 |
| First you filled your jars ALL the way to the top with water |
 |
| Then you added enough water so that the level was slightly higher than the rim of the jar. |
 |
| Then we started adding pennies!! |
 |
| One at a time you added pennies to your already full jar of water to see how many you could get in there before it overflowed. |
 |
| Many of you were able to get quite a few pennies in there before it started to actually over flow!!! |
Now to see what all of this means... we started into how water binds with other water molecules.
Using the models in your cups – make 5 water molecules then place how you think one would bond with 4 others.
What type of bond holds two water molecules together?
Hydrogen Bonds
Today we continued with notes and activities on Water. First refreshing our knowledge of the structure of water and how it bonds with others using two slides from Friday (See above). Then we moved on into Cohesion.Hydrogen Bonds
Next demo was to show cohesion and surface tension using pennies.
 |
| Eventually the pressure would be too much and it would overflow, but the water molecules still are holding on to each other!!!! |
 |
| We balanced a pin on the side of the jar, let go and saw if we could get the pin to float on the water |
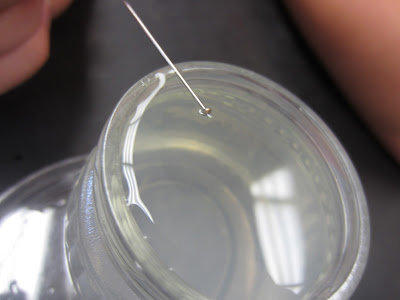 |
| If you look very carefully, you can see how the water slightly "dips" in at where the pin is laying. This is showing the how the hydrogen bonds will "bend" a bit, but it takes a while to break |
Finally you started into Adhesion as we finished up the class period.









No comments:
Post a Comment