
Wednesday, September 18, 2013
Tuesday, September 17, 2013
CDT and first case study
Friday we wrapped up the properties of water with a daily question quiz and you turned in your lab write up. I also had you watch another crash course as an introduction to the next topic of Organic Chemisty.
Yesterday and today you took your baseline CDT. This will give me and you a good look at how you are progressing through the year.
I will be showing you all individually how you did, here is a look at how you scored as a class!!!
After finishing your CDT I had you start into your first case study. As my honors students, I plan to push you guys a bit harder and this year I will be incorporating different case studies as a way to extend you understanding of different topics. This is all research by the national science foundation.
To go along with our section on organic chemistry, you will also be working through the following case study.
Today I had you find a BIOLOGICAL definition of Energy and started looking up the ingredients of different energy drinks.
We will go full force on this tomorrow!!!!
Yesterday and today you took your baseline CDT. This will give me and you a good look at how you are progressing through the year.
I will be showing you all individually how you did, here is a look at how you scored as a class!!!
After finishing your CDT I had you start into your first case study. As my honors students, I plan to push you guys a bit harder and this year I will be incorporating different case studies as a way to extend you understanding of different topics. This is all research by the national science foundation.
To go along with our section on organic chemistry, you will also be working through the following case study.
Today I had you find a BIOLOGICAL definition of Energy and started looking up the ingredients of different energy drinks.
We will go full force on this tomorrow!!!!
Thursday, September 12, 2013
water wrap up
Thursday September 12th
Because water does this it is considered a universal……… Solvent. (Keep in mind though... while it is called this, water CANNOT dissolve EVERYTHING - For instance any it cannot dissolve any Nonpolar substances!!)
Write the equation and diagram out How salt (NaCl) dissolves in water
Water
in solution disassociates into two ions.
What are these two ions? (What caused the charges to happen?)
Because water does this it is considered a universal……… Solvent. (Keep in mind though... while it is called this, water CANNOT dissolve EVERYTHING - For instance any it cannot dissolve any Nonpolar substances!!)
Write the equation and diagram out How salt (NaCl) dissolves in water
In reality this is usually what happens!!!
DAILY QUESTION QUIZ TOMORROW!!!!Specific Heat and Water as a Solvent
Wednesday September 11th
Water will form hydrogen bonds with other substances such as glass, soil and plant tissues. The term for this type of attraction is known as Adhesion
A combination of this and Cohesion can cause water to ‘defy gravity’ by moving UP narrow tubes (such as the roots of trees) in a process known as Capillary Action
**
Water is able to resist drastic changes in temperature because it has a high Specific Heat (heat capacity)
Why is this important to us? (As humans)
We are made up of approximately 75% water as is the EARTH..... We rely on waters ability to store large amounts of heat in order for our planet to remain a livable temperature!!
Speaking of heat capacity..... Today was another brutal hot day, so you guys got to take it RELATIVELY easy. We just discussed specific heat again, moved into the importance of water being LESS dense as a solid than a liquid, and finally about how water is a great solvent. Here were the notes from Wednesday.
We are made up of approximately 75% water as is the EARTH..... We rely on waters ability to store large amounts of heat in order for our planet to remain a livable temperature!!
Speaking of heat capacity..... Today was another brutal hot day, so you guys got to take it RELATIVELY easy. We just discussed specific heat again, moved into the importance of water being LESS dense as a solid than a liquid, and finally about how water is a great solvent. Here were the notes from Wednesday.
Wednesday, September 11, 2013
Adhesion and Capillary Action Notes and activities
Tuesday September 10th
Here is a poor quality video of how we viewed capillary action (pay no attention to the sound ;)
*Water can and WANTS to form bonds with how many other water molecules? 4 (EVERY water molecule wants to bind with 4 others so it will eventually form a crystal like structure like this.
*Waters attraction to other water molecules is known as Cohesion
*The term for how water “pulls in” at the surface to form a “film” is known as Surface Tension (Which we saw yesterday)
A water molecule is a polar molecule used in all living organisms.
Which process is the direct result of the polarity of the water?
A. The storage of chemical energy
B. The destruction of damaged cell parts
C. The transferring of genetic information
D. The dissolving of a variety of substances
We then looked at Adhesion and capillary action today with the following notes and activities.
Here is a poor quality video of how we viewed capillary action (pay no attention to the sound ;)
Monday, September 9, 2013
Wonders of Water Day 2 and 3
Friday we had our daily question quiz and then started into more water labs.
Next demo was to show cohesion and surface tension using pennies.
Next we looked at surface tension with a different demo.......
Finally you started into Adhesion as we finished up the class period.
 |
| First you filled your jars ALL the way to the top with water |
 |
| Then you added enough water so that the level was slightly higher than the rim of the jar. |
 |
| Then we started adding pennies!! |
 |
| One at a time you added pennies to your already full jar of water to see how many you could get in there before it overflowed. |
 |
| Many of you were able to get quite a few pennies in there before it started to actually over flow!!! |
Now to see what all of this means... we started into how water binds with other water molecules.
Using the models in your cups – make 5 water molecules then place how you think one would bond with 4 others.
What type of bond holds two water molecules together?
Hydrogen Bonds
Today we continued with notes and activities on Water. First refreshing our knowledge of the structure of water and how it bonds with others using two slides from Friday (See above). Then we moved on into Cohesion.Hydrogen Bonds
Next demo was to show cohesion and surface tension using pennies.
 |
| Eventually the pressure would be too much and it would overflow, but the water molecules still are holding on to each other!!!! |
 |
| We balanced a pin on the side of the jar, let go and saw if we could get the pin to float on the water |
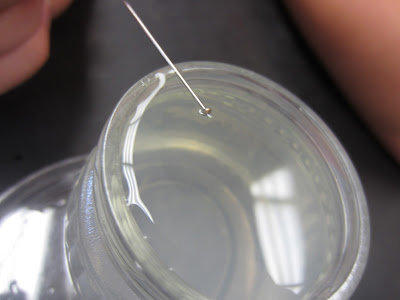 |
| If you look very carefully, you can see how the water slightly "dips" in at where the pin is laying. This is showing the how the hydrogen bonds will "bend" a bit, but it takes a while to break |
Finally you started into Adhesion as we finished up the class period.
Thursday, September 5, 2013
Wonders of water day 1
Thursday September 5th
Based on this cartoon – What type of Bond is this “marriage” depicting since the guy states “I Do! Here’s my Valence Electron Baby” Ionic (In ionic bonds electrons are lost or gained, this causes charges and then the attraction of a negative ion to a positive ion.)
What if instead the “boy” atom said, “I do, Let’s share our valence electrons baby” Which type of bond would it be referring to? Covalent. When electrons are SHARED, this is known as a covalent bond (which makes for a MUCH better relationship ;)
We reviewed the last couple slides from yesterday and then started into the following notes/activities.
 |
| I had your cups of happy water ready on your desk for you..... as we stared our first slide |
 |
| Then we built our first water molecule |
 |
| The large red atom is the oxygen, the small white atoms are hydrogen. |
 |
| You then made smaller water molecules to see how water interacts with other water molecules |
I had you guess, based on your understanding of water structure, how two water molecules would bond with each other.
 |
| Most of you were able to infer that the hydrogen end of one water molecule would be attracted to the negative end of another. |
Subscribe to:
Posts (Atom)












































